Partner with Tekton Research
Your Site Network Partner for Executing Phase 1-4 Clinical Trials.
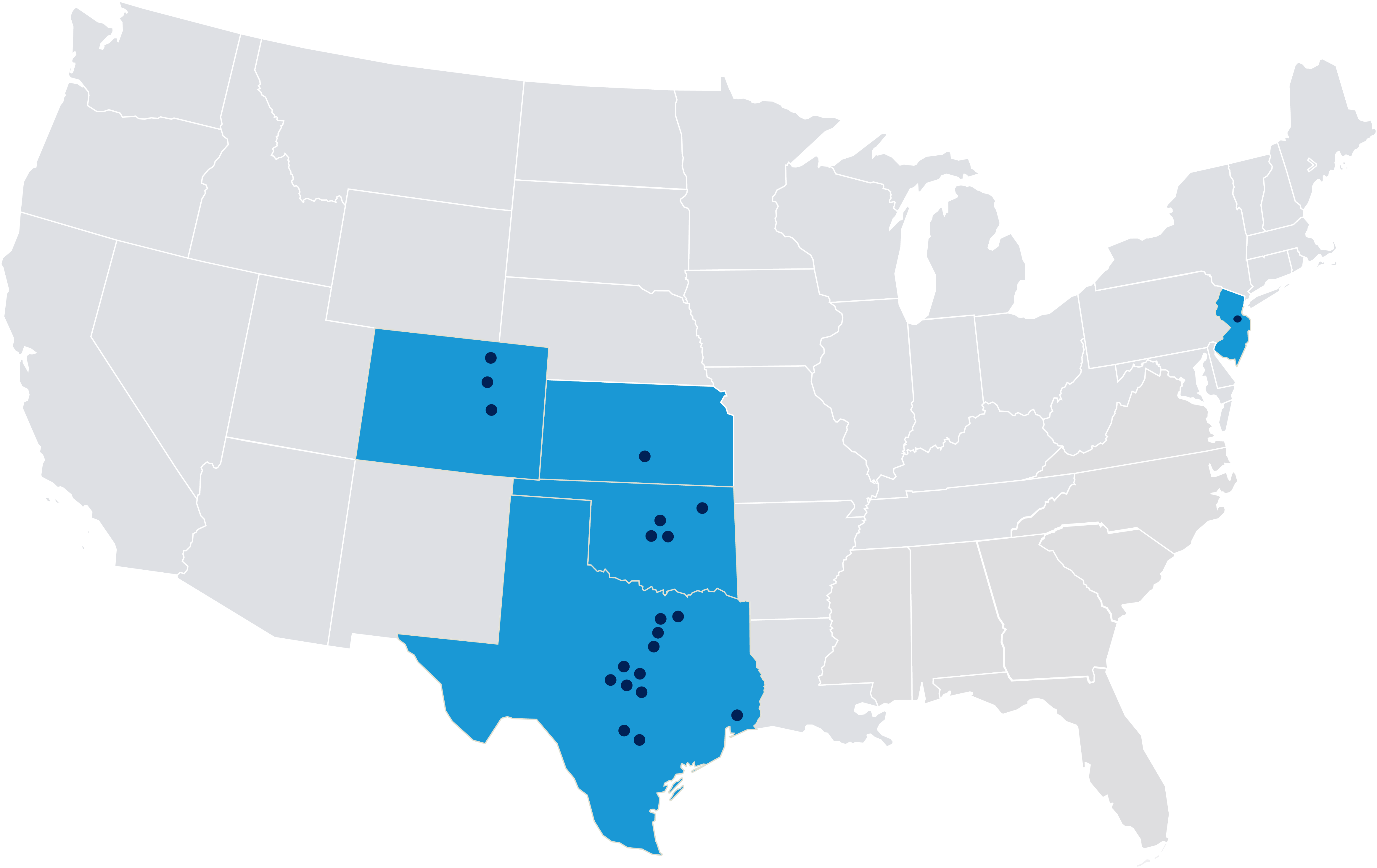
Welcome to Tekton Research, your premier partner for conducting Phase 1-4 clinical trials with unparalleled excellence. Our extensive investigator network covers key locations in Colorado, Kansas, New Jersey, Oklahoma, and Texas, showcasing exceptional expertise and unwavering commitment throughout the entire research process.
Backed by a team of accomplished principal investigators with diverse experience across therapeutic areas such as Endocrinology, Neurology, Dermatology, Rheumatology, and more, we successfully engage pediatric, adult, maternal, and elderly populations in our recruitment efforts.
Multi-Specialty
Therapeutic Areas

Hypertension
Hypercholesterolemia
Acne
Diabetes
Obesity
Osteoarthritis
Osteoporosis
Depression
Healthy Vaccine Studies
Treatment Vaccine Studies
Device Studies

Alzheimer’s disease
Amyloidosis
Depression
Epilepsy, idiopathic generalized epilepsy (IGE)
Essential Tremors
Ischemic stroke
Memory impairment
Migraine
Mild cognitive impairment (MCI)
Multiple sclerosis
Pain management
Parkinson’s disease
Restless legs syndrome
Psoriatic Arthritis
Lupus
Sjogren’s Syndrome
Ankylosing Spondyloarthritis
Osteoarthritis
Osteoporosis
Gout
Giant Cell Arteritis
Mixed Connective Tissue Disease
PMR
Scleroderma
Fibromyalgia
Type 2 Diabetes
Obesity
Hypothyroidism/Hyperthyroidism
Lipids
Hypertension
PCOS
Osteoporosis
Growth Hormone Deficiencies
Parathyroid Disorders
Thyroid Disorders
Pituitary Disorders
Adrenal Gland Disorders
Fatty Liver
Thyroid Disease
Obesity
Device Studies
Low Testosterone
Metabolic disorders
Osteoporosis
Influenza
Maternal
Newborns and Infants
Pediatrics, Adolescents
Geriatrics
Immunodeficient
MMR (Measles, Mumps, Rubella)
mRNA Vaccines
Norovirus
Parainfluenza
Pediatric Influenza
Pediatric Meningitis
Pertussis (Whooping Cough)
Plague
Pneumococcal Disease
Rabies
Respiratory Syncytial Virus
Smallpox
Staphylococcus
Tetanus
Type 3 Parainfluenza Virus (PIV3)/RSV Combo
Varicella
West Nile Virus
Yellow Fever
Zika Virus
Anthrax
Avian Influenza
Botulinum
C. difficile (C-diff)
Celiac Disease
Chikungunya
COVID-19 (SARS-CoV-2)
Cytomegalovirus
Dengue Fever
Diphtheria
Ebola
E. coli
Encephalitis
Epstein-Barr Virus (EBV)
Group B Streptococcus
Hepatitis B
Herpes Zoster (Shingles)
HIV
Human Metapneumovirus
Human Papillomavirus
Influenza (H1N1, H5N1, H7N1, H7N9)
Lyme Disease
Meningococcal Disease

Urine
Stool
Tissue
Saliva

Hypertension
Hypercholesterolemia
Acne
Diabetes
Obesity
Osteoarthritis
Osteoporosis
Healthy Vaccine Studies
Treatment Vaccine Studies
Device Studies

Actinic keratosis
Alopecia
Atopic dermatitis/eczema
Cellulite
Consumer products
Cosmetics
Fine Lines
Granuloma annulare
Hair loss
Hidradenitis suppurativa
Histology
Hyperhidrosis
Hyperpigmentation
Impetigo
Infections
Melanoma and skin cancers
Mid-face filler
Molluscum contagiosum
Nail fungus
Onychomycosis
Palmoplantar Pustulosis
Photoaging
Prurigo Nodularis
Pruritus and urticaria
Psoriasis
Purpura
Rosacea
Seborrheic dermatitis
Shingles
Surgical products
Tinea pedis/onychomycosis
Vitiligo
Warts
Wound care and scars

Cytomegalovirus
Endometriosis
Fetal health
Group B Streptococcus
Herpes
High-grade squamous intraepithelial lesion (HSIL)
Hot flash
Human papillomavirus (HPV) vaccines
Maternal vaccine
Minimally-invasive surgical procedures
Osteoporosis
Overactive bladder
Sexually-transmitted diseases
Urinary incontinence
Uterine fibroids
Vulvo-vaginal atrophy (VVA), dryness

Diagnostic Device Studies
Sample Collection Studies
Vaccine Studies

COVID-19 vaccine
Epilepsy
H5N1, H1N1 influenza vaccine
HPV vaccine
Human metapneumovirus (HMPV) vaccine
Infant formula
Influenza treatment
Lyme disease
Maternal vaccines
Measles, mumps, and rubella vaccine
Meningococcal vaccine
Migraines
Molluscum contagiosum
On- and off-season influenza vaccine
Parainfluenza virus vaccine
Pneumococcal vaccine
RSV vaccine
Tetanus, diphtheria, pertussis (Tdap) vaccine
Computed tomography (CT) scan
Echocardiography
Exercise stress testing
Magnetic resonance angiography (MRA)
Magnetic resonance imaging (MRI)
Multigated acquisition scan (MUGA) imaging
Positron emission tomography (PET) pharmacologic and stress/rest perfusion, as well as metabolic imaging
Pharmacologic stress testing
Stress echocardiography
Technetium 99m stress/rest imaging
Thallium 201 stress/rest imaging
Transesophageal echocardiography (TEE)
Cardiac MRIs
CPET (exercise stress testing)
Our Site Network
Locations
We Manage the Study Lifecycle from Start to Finish
Capabilities
- Startup Efficiencies
- Feasibility and Site Selection
- Patient Recruitment, Engagement, and Retention
- Quality Assurance
Startup Efficiencies
Tekton sites aim to be your first activated and enrolling sites. Our dedicated regulatory and contract teams collaborate with our clinical operations and recruitment departments, so we can efficiently activate our sites and begin screening upon green light.
Our clients have a single point of contact that oversees all start-up activities. Tekton Research utilizes metrics to ensure all action items remain on track to achieve target study timelines while keeping our clients informed about progress or barriers.
Feasibility and Site Selection
Tekton’s feasibility team and investigators are committed to providing reliable and timely feedback for site selection. Our sites can operate under a single confidentiality agreement or execute site-specific agreements based on client requirements. Our goal is to supply our clients with an enriched and accurate list of well-aligned, experienced sites that understand the indication and protocol complexities.
Tekton will propose PI’s and sites that are engaged, invested, and dedicated to meeting enrollment goals, providing a positive patient experience, and delivering quality data.
Patient Recruitment, Engagement and Retention
At Tekton, we begin considering the overall patient recruitment, engagement, and retention strategy once an initial feasibility questionnaire is received. Our patient recruitment department, operational team, and site staff work together, considering: patient populations, previous best practices, the complexities of the indication, inclusion and exclusion criteria, study design, and goals, to develop an efficient and effective patient recruitment strategy. A Tekton patient recruitment strategist collaborates with our clients and internal stakeholders to develop and execute these patient recruitment plans. We understand the importance of establishing a well-qualified patient pool as early as possible.
The Tekton team employs a variety of resources to pre-identify participants, including investigator databases, Tekton databases, community outreach and advocacy, local and centralized advertising campaigns, and AI technology. Our clinical site staff and dedicated recruitment specialists connect frequently with current study subjects as well as our Tekton proprietary databases, ensuring they remain interested, informed, and engaged. This communication improves the likelihood of future enrollment and dramatically improves patient retention for enrolling trials. Tekton collaborates with site staff, providers, and participants to actively involve patients in research, prioritizing their perspectives and needs, to provide the best patient experience possible.
Quality Assurance
Tekton sites adhere to SOPs and quality assurance initiatives in compliance with corporate and industry guidelines. Overseen by a dedicated QA team, Tekton conducts regular training sessions and updates within our quality management system.
Processes and practices are reviewed regularly to stay aligned with GCP, ICH, and FDA regulations. Being audit-ready at all times demonstrates Tekton’s commitment to excellence and regulatory compliance.
Read Our Latest News
 Tekton Research Collaborates with Leading Neurologist Dr. Amor Mehta for Clinical Research Expansion in New Jersey
Tekton Research Collaborates with Leading Neurologist Dr. Amor Mehta for Clinical Research Expansion in New Jersey
 Tekton Research Announces Strategic Partnership with Dr. Alex Reish to Advance CNS Clinical Research
Tekton Research Announces Strategic Partnership with Dr. Alex Reish to Advance CNS Clinical Research
Tekton Research Announces Strategic Partnership with Dr. Alex Reish to Advance CNS Clinical Research
 Understanding Influenza: The Vital Role of Research in Vaccine Development
Understanding Influenza: The Vital Role of Research in Vaccine Development
Understanding Influenza: The Vital Role of Research in Vaccine Development
 Tekton Research Forms Strategic Partnership with Esteemed Family Medicine Specialist Dr. Corey Chamness in Tulsa, Oklahoma
Tekton Research Forms Strategic Partnership with Esteemed Family Medicine Specialist Dr. Corey Chamness in Tulsa, Oklahoma
Tekton Research Forms Strategic Partnership with Esteemed Family Medicine Specialist Dr. Corey Chamness in Tulsa, Oklahoma
 Tekton Research and Dr. Martha Arambula Partner to Lead Pediatric Research Advancements in Yukon, OK
Tekton Research and Dr. Martha Arambula Partner to Lead Pediatric Research Advancements in Yukon, OK
Tekton Research and Dr. Martha Arambula Partner to Lead Pediatric Research Advancements in Yukon, OK
 Tekton Research Appoints Jeffrey Zucker as Vice President of Neurology Operations
Tekton Research Appoints Jeffrey Zucker as Vice President of Neurology Operations
Tekton Research Appoints Jeffrey Zucker as Vice President of Neurology Operations
Our Team
Our staff operates cohesively as a team, fostering collaboration and unity. We emphasize a collaborative relationship with our clients, ensuring that we work together seamlessly to achieve shared goals.

Kip championed bold initiatives to build a premier and sought-after site network for vaccine and specialty research. His business acumen led him to develop a strategic and unique business model that allows the company to grow organically and steadily with sustainable financial risk.

Corey’s early career included various financial leadership roles at GE Water & Process Technologies and Deloitte. He earned his bachelor’s degree in accounting from the University of South Carolina and is a Certified Public Accountant in the Commonwealth of Pennsylvania.
Corey is already working diligently to position Tekton for growth.

Bruce has had an extensive career in the pharmaceutical and clinical research industries, having recently served as Chief Commercial Officer at Emvenio Research. Prior to this role, he was Senior Vice President, Clinical Trials, Global Head of Commercial at Matrix Medical Network, and Vice President, Life Sciences Business Development, at Inteliquet. Bruce held senior roles at several other leading industry organizations, where he demonstrated proficiency in building strategic partnerships and driving revenue growth across different phases of drug development.
Bruce holds a Bachelor of Science degree in Business Management from the University of North Carolina, Wilmington. Today, he lives in St. Petersburg, Florida, with his wife, Lara, and three daughters.

Jeff resides in Philadelphia and holds a Bachelor of Science from Penn State University and a Masters of Psychology from Hahnemann University. His extensive experience spans multiple facets of the industry.
Jeff began his career as a psychotherapist, working with diverse populations in various settings. He transitioned to clinical research in 1996, managing a research facility focused on Neurology and Psychiatry in Princeton, NJ. At Eli Lilly, he established and nurtured site relationships and contributed to the development of CNS protocols to ensure their feasibility.
As a consultant, Jeff opened new sites, expanded existing ones, and managed a phase I unit. In the CRO space, he spent 13 years at Worldwide Clinical Trials, serving as SVP of Clinical Operations for 8 years. In this role, he provided oversight for Site Management, Rater Training, Feasibility, Patient Recruitment, and Decentralized Clinical Trials departments.

Alison’s passion is diabetes and metabolic disease. Alison is currently the Executive Director of Operations at Tekton Research overseeing all operations in five states with a heavy focus on vaccine research.

She now oversees Tekton’s business development team, feasibility operations, and strategic client relationships. Blaire serves as a central point of contact for all Tekton sites and clients and is determined to build win-win relationships with our clients and partners.

In 2020 and 2021, Taryn played a crucial role in managing COVID-19 vaccine trials, overseeing studies that contributed to vaccines receiving Emergency Use Authorization from the FDA. This work underscored Taryn's commitment to advancing medical research during critical times.
In 2023, [Your Name] transitioned into a Director of Quality Assurance position for a network of 23 sites. In this role, [Your Name] developed and implemented a comprehensive QA program, ensuring the highest standards of quality and compliance across the network.

Request a Meeting
"*" indicates required fields